Background
Crovalimab is a novel anti-human complement component 5 (C5) antibody currently under investigation as a therapy for paroxysmal nocturnal hemoglobinuria (PNH), a life-threatening disorder characterized by hemolytic anemia and thrombosis. In the Phase I/II COMPOSER trial (NCT03157635; Röth et al. Blood. 2020), crovalimab showed promise as a therapy for PNH in patients with or without prior treatment with C5 inhibitors. Eculizumab and ravulizumab are C5 inhibitors currently approved for the treatment of patients with PNH, yet treatment limitations remain. Some patients experience breakthrough hemolysis due to unsustained C5 inhibition, there may be a lack of efficacy in patients with C5 mutational variants, and the requirement for regular intravenous (IV) infusions contributes to the treatment burden. Crovalimab is engineered to have a significantly extended half-life, enabling subcutaneous (SC) administration once every 4 weeks (Q4W), which could significantly reduce the treatment burden on patients with PNH.
Study Design and Methods
The Phase III, randomized, open-label, active-controlled, multicenter COMMODORE 1 study (NCT04432584) is evaluating the efficacy and safety of crovalimab compared with eculizumab in adult and adolescent patients with PNH currently treated with complement inhibitors.
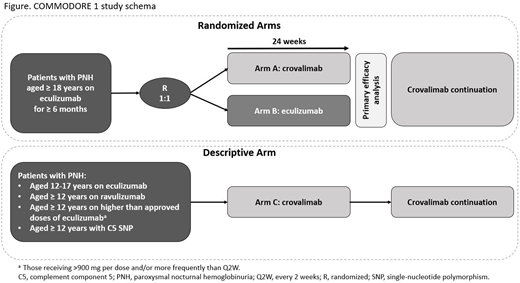
The study design is divided into 2 parts: randomized arms (Arms A and B) for primary and secondary efficacy analyses and a descriptive arm (Arm C) for exploratory analysis in patients of clinical relevance and importance (Figure). Adult patients with PNH (aged ≥ 18 years) are randomized 1:1 to receive either crovalimab (Arm A) or eculizumab (Arm B). The crovalimab regimen includes a loading series of an IV dose on Day 1 followed by weekly SC doses for 4 weeks starting on Day 2. This is followed by SC Q4W maintenance dosing starting at Week 5. Arm B patients receive IV maintenance dosing starting on Day 1 and then once every 2 weeks (Q2W) for 24 weeks. The descriptive analysis arm (Arm C) patients will receive crovalimab (same dosing regimen as Arm A patients). It will include:
1. Adolescent patients (aged 12-17 years) currently treated with eculizumab
2. Patients (aged ≥ 12 years) currently treated with ravulizumab
3. Patients (aged ≥ 12 years) currently treated with eculizumab at a dose > 900 mg and/or more frequent than Q2W
4. Patients (aged ≥ 12 years) with a known C5 polymorphism whose hemolysis was poorly controlled
After 24 weeks of treatment, patients from each treatment arm can continue crovalimab or switch from eculizumab to crovalimab if their physician determines this is in their best interest.
The primary efficacy objective for the randomized arms is to determine the noninferiority of crovalimab compared with eculizumab based on mean percent change in lactate dehydrogenase (LDH) levels from baseline to after 24 weeks of treatment. Secondary efficacy objectives are to determine the noninferiority of crovalimab compared with eculizumab based on (1) proportion of patients who achieve transfusion avoidance, (2) proportion of patients who experience breakthrough hemolysis, (3) proportion of patients who achieve stabilization of hemoglobin, and (4) mean change in fatigue as assessed by the Functional Assessment of Chronic Illness Therapy-Fatigue questionnaire. The safety objective is to evaluate the safety and tolerability of crovalimab compared with eculizumab based on the incidence and severity of adverse events, including infections (meningococcal meningitis and other infections), injection-site reactions, infusion-related reactions, hypersensitivity, adverse events leading to study drug discontinuation, and type 3 hypersensitivity reactions in patients who switch to crovalimab from eculizumab or ravulizumab. Pharmacokinetic, immunogenicity, biomarker, and health status utility objectives will also be assessed.
Risitano:Amyndas: Consultancy; Samsung: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Alnylam: Research Funding; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Jazz: Speakers Bureau; RA pharma: Research Funding; Biocryst: Membership on an entity's Board of Directors or advisory committees; Apellis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Speakers Bureau; Achillion: Membership on an entity's Board of Directors or advisory committees. Röth:Biocryst: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria, Research Funding; Apellis: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding. Kulasekararaj:Alexion Pharmaceuticals Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pu:F. Hoffmann-La Roche Ltd: Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland; Pennsylvania State University: Patents & Royalties; SUNY Upstate Medical University: Current Employment. Nishimura:Chugai: Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Membership on an entity's Board of Directors or advisory committees, Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Wright:Genentech, Inc: Current Employment; F. Hoffmann-La Roche Ltd: Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Appius:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Sostelly:F. Hoffmann-La Roche Ltd: Current Employment, Other: All authors received support for third-party writing assistance, furnished by Scott Battle, PhD, provided by F. Hoffmann-La Roche, Basel, Switzerland.. Sreckovic:F. Hoffmann-La Roche Ltd: Current Employment, Other: All authors received support for third-party writing assistance, furnished by Scott Battle, PhD, provided by F. Hoffmann-La Roche, Basel, Switzerland.. Vignal:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Munir:F. Hoffmann-La Roche: Consultancy, Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland; Alexion: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal